Ionic Liquids and High Temperature Molten Salts
We are interested in understanding the structure, dynamics, spectroscopy and reactivity of these systems.

Salts are important for many technological problems such as the recovery rare earth elements and molten salt reactors. Our group is interested in understanding the liquid landscape (how do ions organize in the molten state). We do this by developing ways of predicting and analyzing scattering experiments done at synchrotrons.
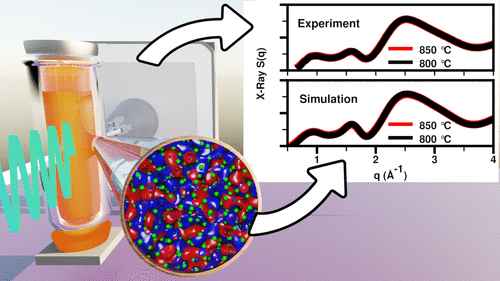
The figure above is the graphical TOC from article "Complete Description of the LaCl3–NaCl Melt Structure and the Concept of a Spacer Salt That Causes Structural Heterogeneity" J. Am. Chem. Soc. 2022, 144, 47, 21751–21762.
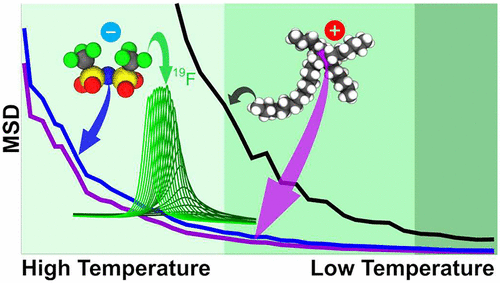
We are also interested in understanding the dynamics of ions and how it relates to structure. The figure below from article "Do Ionic Liquids Slow Down in Stages?" J. Am. Chem. Soc. 2023, 145, 47, 25518–25522 exemplifies how charge networks and apolar domains may have significantly different short-time dynamics.
And then there is the question of how is charge transport (conductivity) different in liquids only made of ions as compared to electrolyte solutions and how is the viscosity of ionic liquids related to structure. We address such type of questions here: How Is Charge Transport Different in Ionic Liquids and Electrolyte Solutions? J. Phys. Chem. B 2011, 115, 45, 13212–13221
and here: A Pictorial View of Viscosity in Ionic Liquids and the Link to Nanostructural Heterogeneity J. Phys. Chem. Lett. 2020, 11, 6, 2062–2066
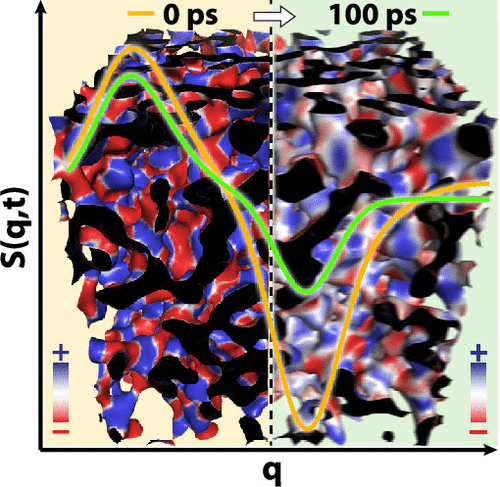
We find that viscosity has much to do with the dynamics of the charge network, as the figure above highlights.
We are part of the MSEE Energy Frontier Research Center working on Molten Salts.
We are also part of two other teams working on excess electrons in ionic liquids and on ionic liquid structural dynamics.